USP 797 Compliance Cleanroom Cleaning Supplies and Apparel
Cleanroom Connection is the leading cleanroom supplier that specializes in USP 797 compliance. We not only stock all of the proper USP 797 compliant cleanroom apparel and cleaning products, but also help you pick the correct cleanroom products. Our cleanroom consultants also explain how to use them. We also offer USP 797 compliance cleanroom consulting and training by industry-leading expert pharamcist consultants with over 25 years experience managing the nation’s leading pharmacy clean rooms.
USP 797 compliance requires that the preparation of pharmaceuticals occur in ISO Class 5 (Class 100) clean room with a buffer area of ISO Class 7 (Class 10,000) plus ante-areas of at least ISO Class 8 (Class 100,000). Cleanroom Connection supplies cleaning products specifically designed for use in clean areas of compounding pharmacies, hospitals, surgery centers and other medical facilities. Our US Pharmacopoeia (USP) compliant pharmacy clients include compounding pharmacies, hospitals and other medical environments. Our products are used to clean and sanitize clean rooms and labs. Our USP 797 compliance consultants can assist you with selection of products that will support your staff to prevent microbial contamination, to control intended strength of ingredients, and eliminate introduction of endotoxins or unintended chemical or physical contaminants. Whether compounding hazardous drugs or working with low-risk compounded pharmaceuticals, our cleanroom supplies can support your controlled environment.
For the best service, please call to speak with our USP 797 experts to get advice on which cleanroom supplies and apparel to choose. We specialize in USP 797 compliance and have helped over 500 hospitals and pharmacies become USP 797 compliant. Our cleanroom consultants can also assist with USP 800 compliance for working with hazardous drugs.
View The Major 2023 USP 797 Updates Below
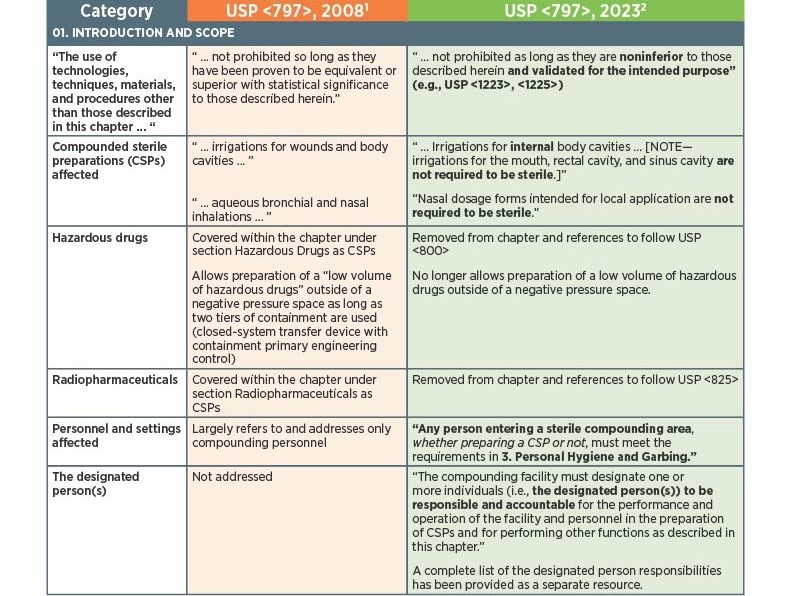
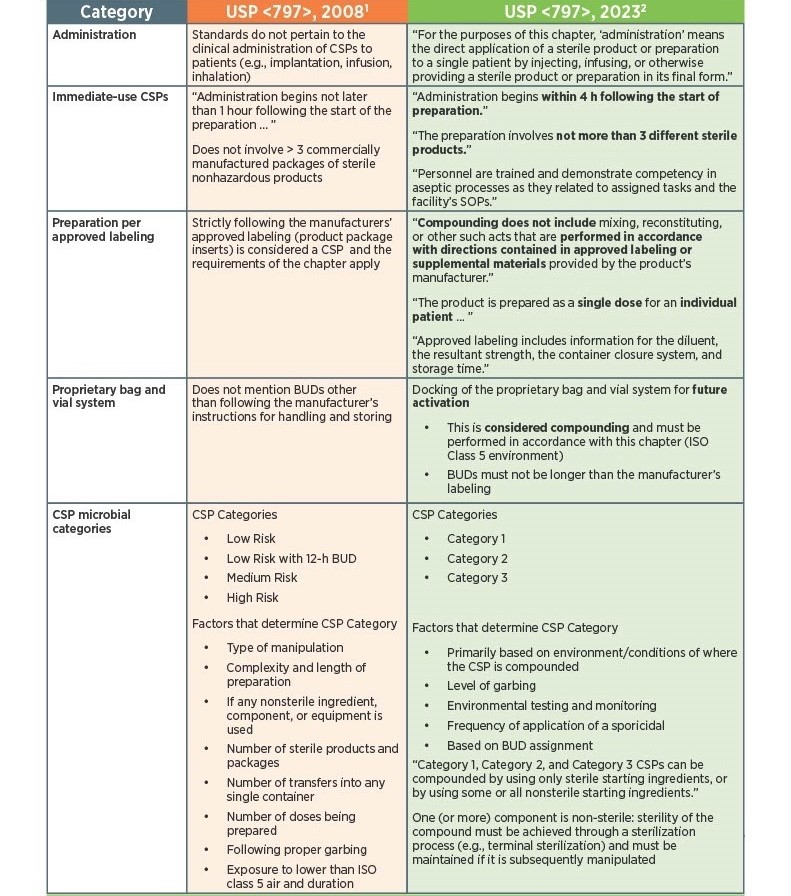
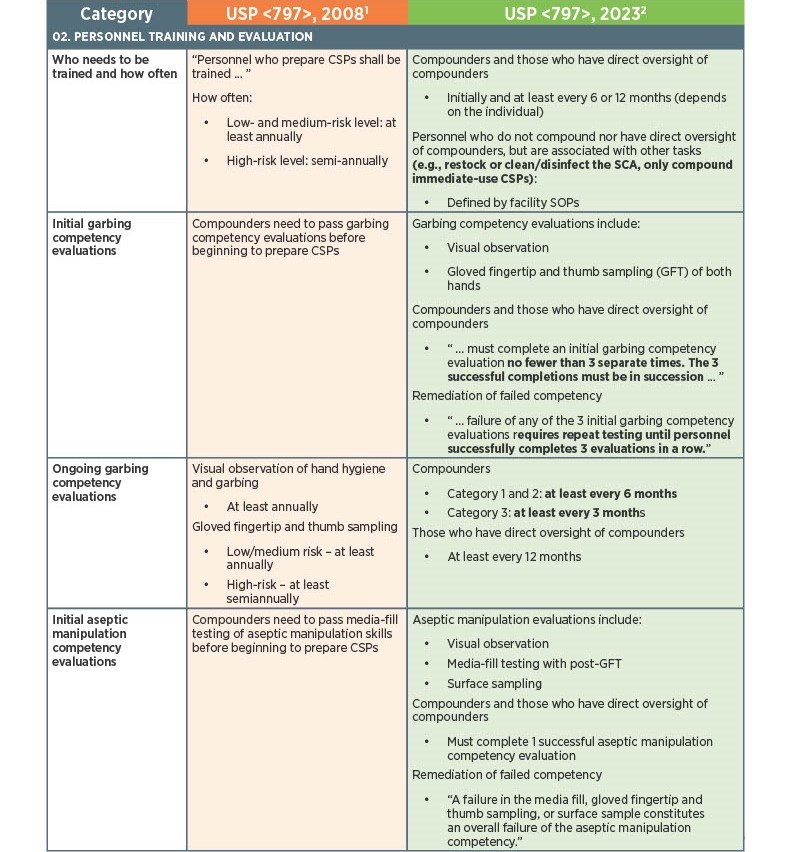
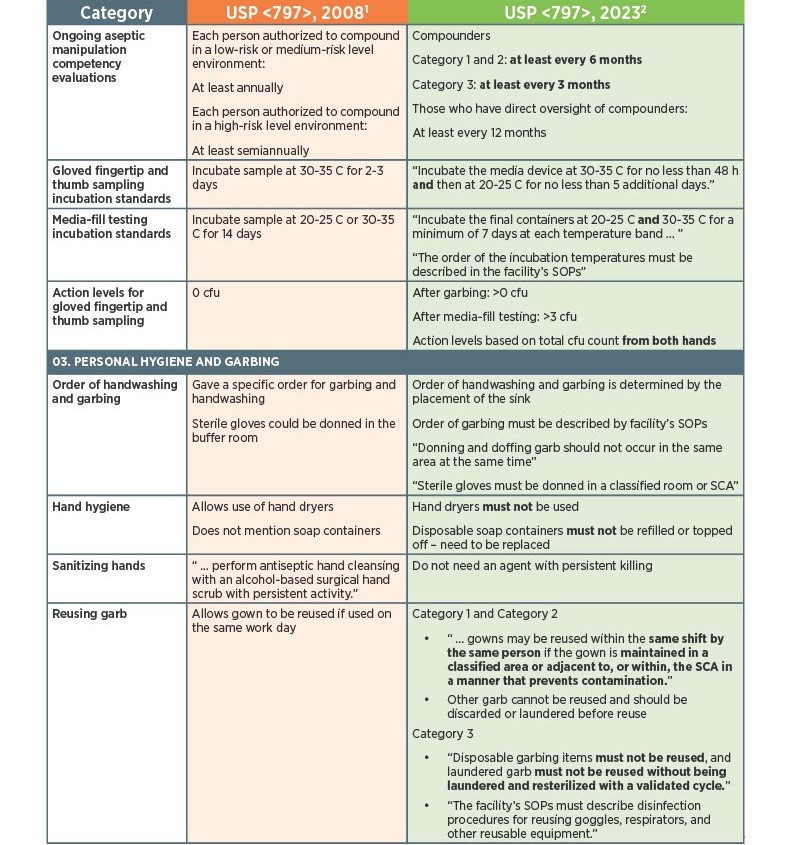
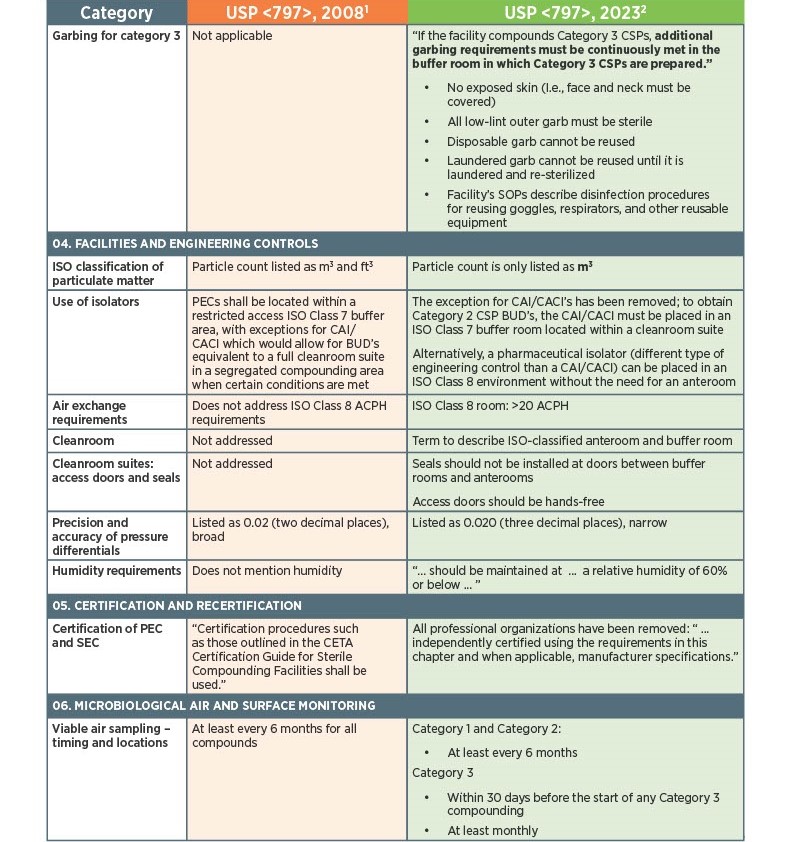
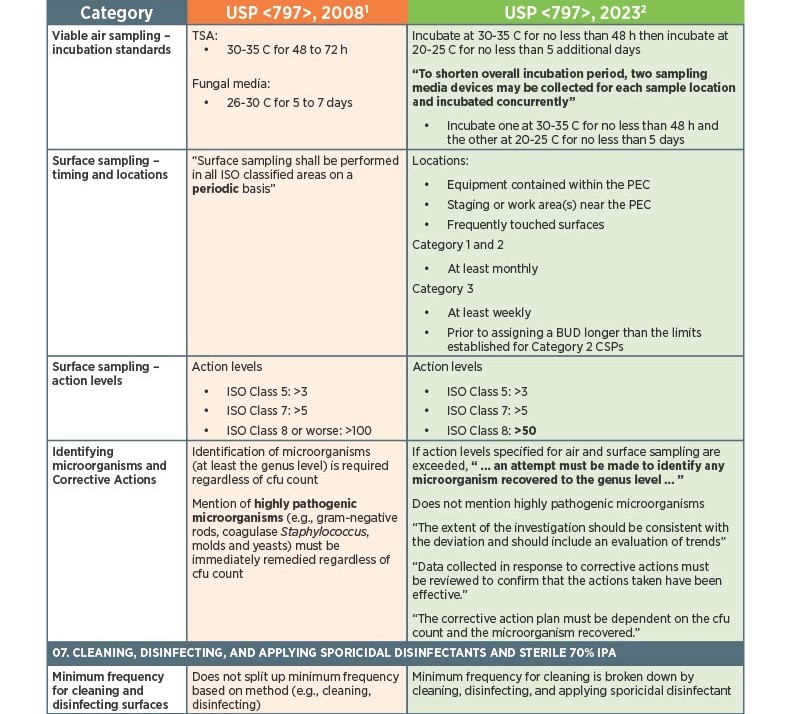
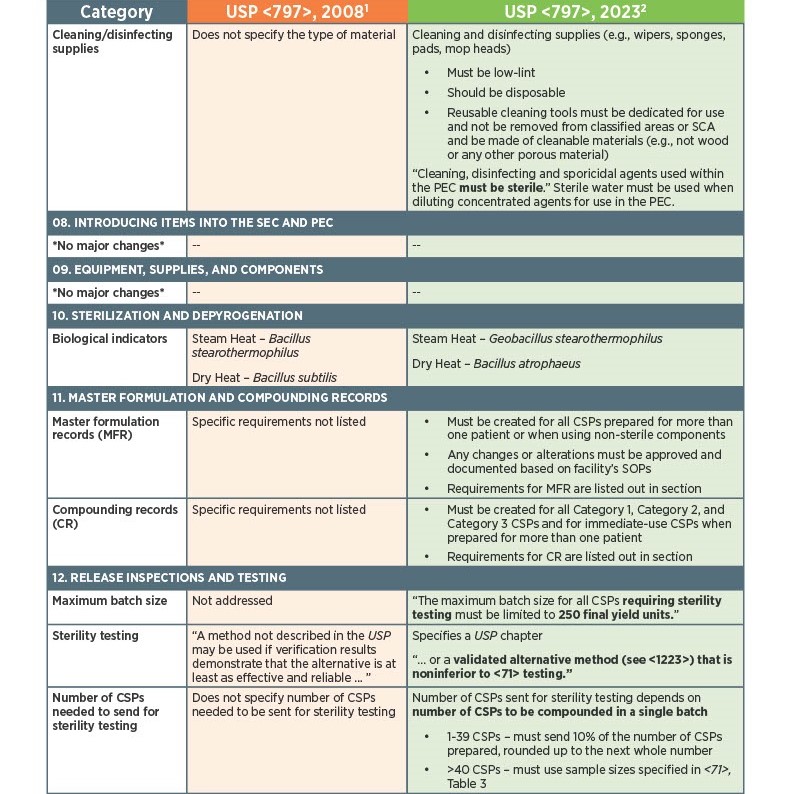
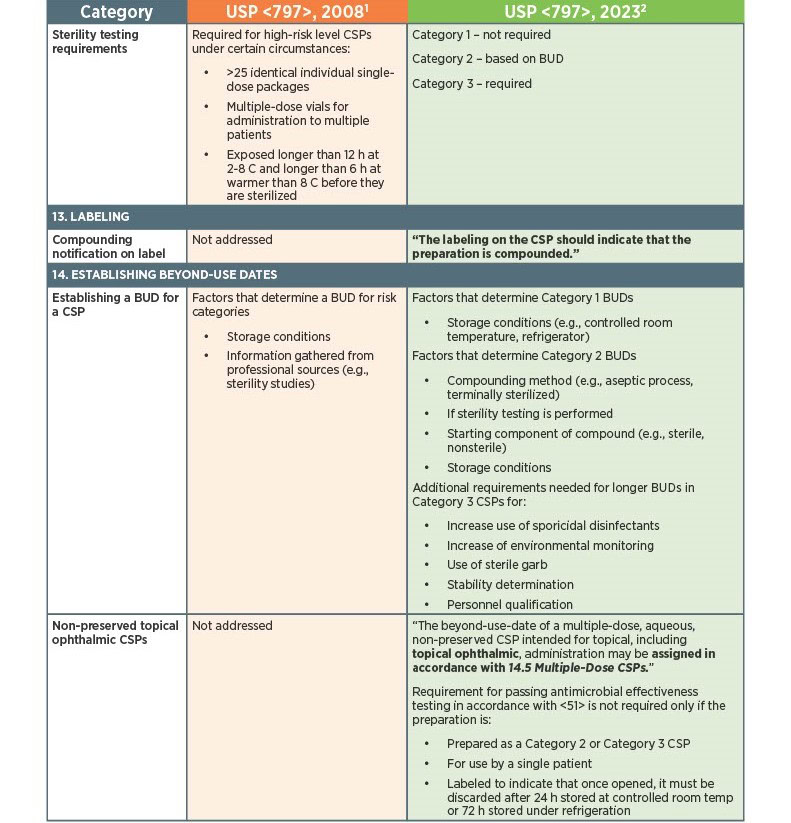
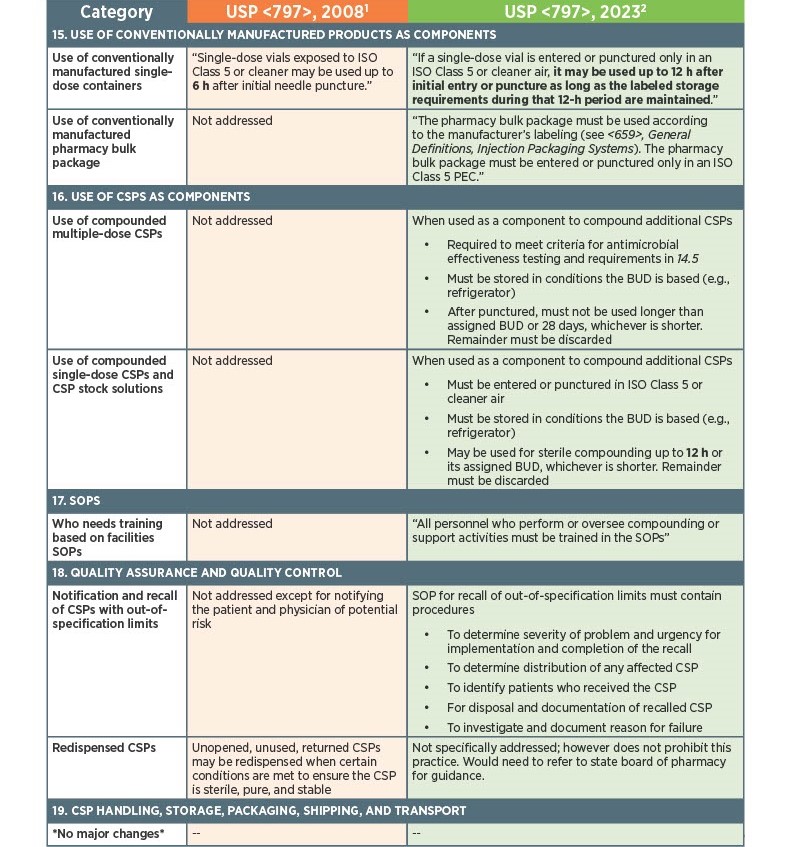
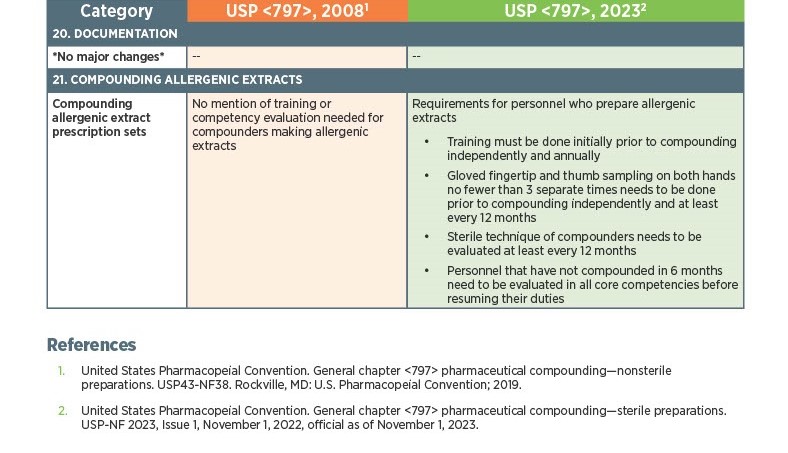
As of November 1, 2023, there have been key changes in the enforceable version of USP Chapter <797> compliance. There are many changes, however below there is a list below of the key updates that we think our sterile compounding pharmacy customers should be aware of. Some of the updates that are listed below are direct text excerpts from the respective chapter which are notated by quotation marks. The others are listed as general comments describing the text or change. Some items are in bold text, and are more major points.