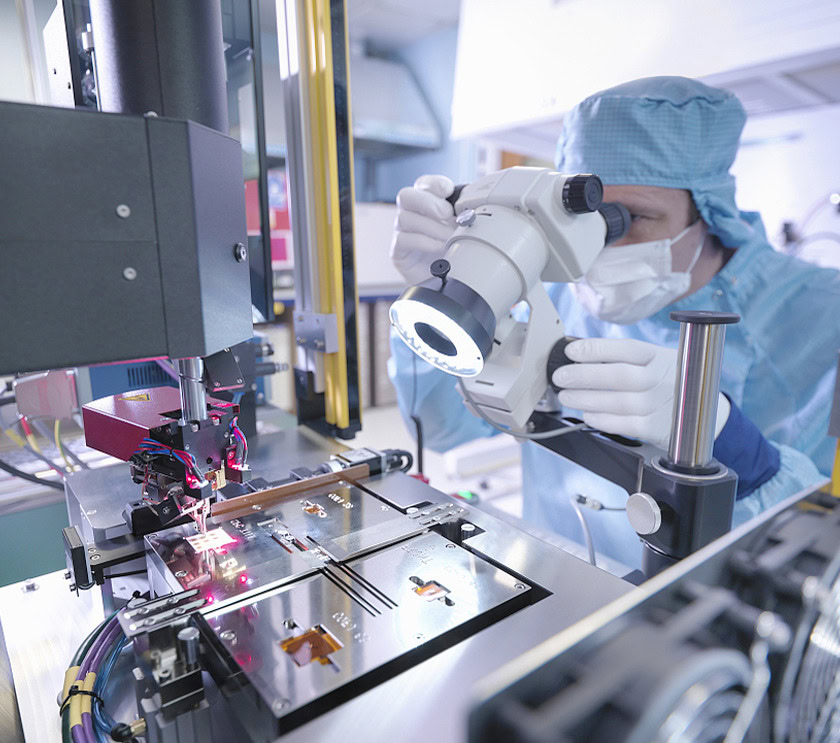
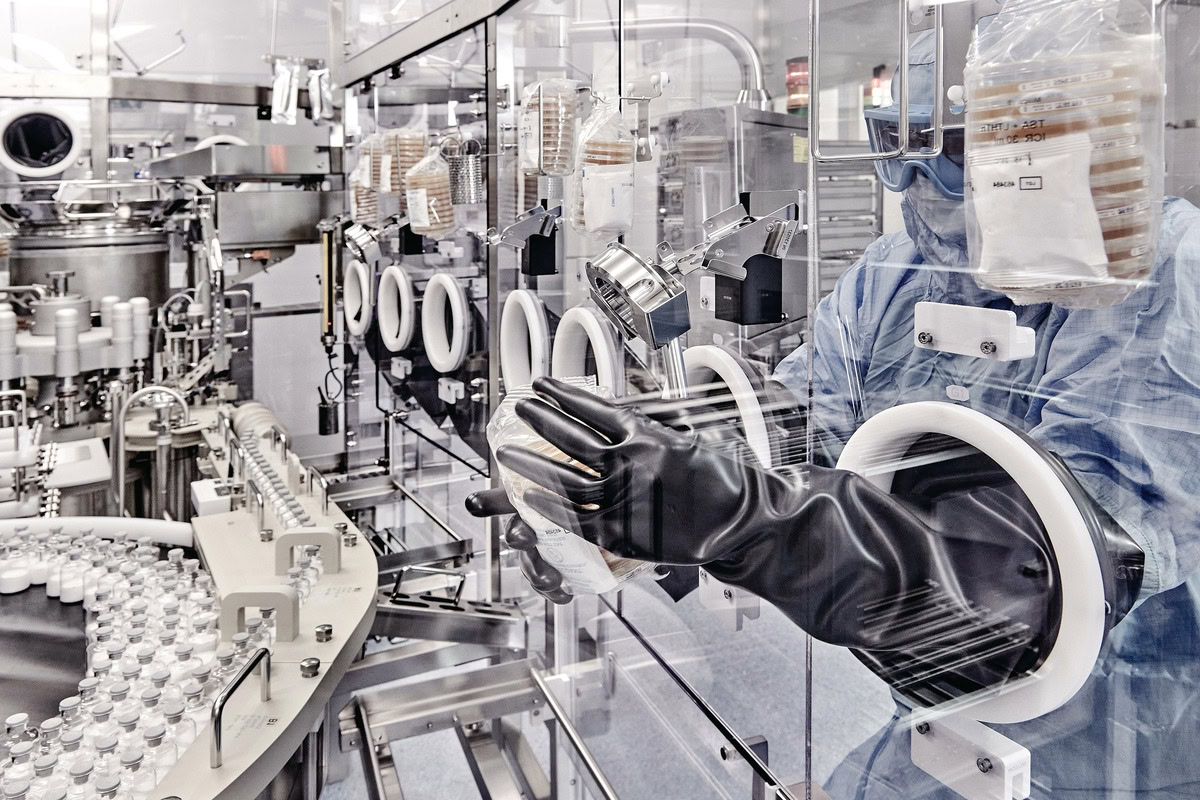
In an era where breakthroughs in biotechnology and life sciences are reshaping our understanding of health and the environment, the need for contamination-free research spaces has never been more crucial. Cleanrooms play a pivotal role in ensuring that sensitive processes remain unaffected by external variables, providing a controlled environment tailored for rigorous scientific explorations.
These specialized facilities are governed by strict ISO classifications that dictate airborne particle levels, humidity, and temperature, all vital for maintaining purity in research outputs. Cleanroom products and cleanroom apparel are a main way to achieve these strict standards. Given the aggressive pace of innovation in medical, agricultural, and environmental biotechnology, cleanrooms are indispensable to ensure high-quality products and adherence to safety protocols.
This article will delve deeper into how cleanrooms enhance research in biotech and life sciences, exploring their essential features, applications, and the future of cleanroom technology. From ensuring product quality to addressing emerging challenges, the role of cleanrooms is fundamental in driving the next wave of scientific advancement.
Key Features of Cleanrooms
Cleanrooms are critical for maintaining a safe and contamination-free environment in the life sciences and biotechnology sectors. These facilities incorporate specialized air filtration systems, including HEPA filters with 99.99% efficiency on 0.3-micron particles, to minimize contamination. Compliance with international standards like ISO 14644 and Good Manufacturing Practices (GMP) is essential in designing and constructing these environments for biotechnology and pharmaceutical applications.
Controlled Environment
A controlled environment in cleanrooms effectively minimizes particulate and microbial contamination. This is crucial for maintaining sterility in biotechnology and life sciences research. Cleanrooms utilize physical barriers such as seamless partitions, ceilings, and floors to separate classified from unclassified areas, preventing contamination. Airflow and filtration systems meet strict ISO standards, including Class 5, to maintain air purity and controlled air velocity.
ISO Classifications
Biotech cleanrooms must adhere to the ISO 14644-1 classification, with Class 5 standards being common to ensure high air cleanliness. These classifications range from Class 1, the cleanest, to Class 9, and dictate the allowable level of airborne particles. Class 5 cleanrooms allow no more than 3,520 particles measuring 5 µm or larger and require airflow rates of 40 to 80 feet per minute. Hardwall construction is preferred for biotechnology cleanrooms to provide superior protection against contamination. Class 100 sterile cleanroom wipes and lint-free sterile cleanroom mops are often used to maintain these strict ISO classifications. They are manufactured using specialized materials for strict cleanliness. Depending on your ISO cleanliness classification, Cleanroom Connection can recommend the correct cleanroom supplies to maintain the environment.
Importance of Cleanrooms in Biotech and Life Sciences
Cleanrooms are critical in the biotech and life sciences industries, providing the necessary controlled environments for producing biologics like vaccines and gene therapies. Their design aims to minimize airborne particles, thereby protecting sensitive processes integral to pharmaceuticals and life sciences. By employing advanced systems such as multi-stage HEPA filtration and laminar airflow, cleanrooms maintain the stringent air quality control needed for biotech operations. Compliance with Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP) is facilitated through the use of these specialized facilities, ensuring the production of reliable scientific data and high-quality products.
Ensuring Product Quality
The role of cleanrooms in ensuring product quality is paramount, as they significantly reduce the risk of contamination throughout manufacturing, packaging, and storage processes. This controlled environment is essential for maintaining the safety and efficacy of pharmaceuticals and biotechnology products, directly impacting patient health. By adhering to strict industry standards and incorporating humidity control as well as air filtration, cleanrooms safeguard product stability and the reproducibility of research results in the life sciences sector. When handling sensitive or sterile products in your cleanroom, we recommend to wear sterile cleanroom gloves along with wearing a sterile cleanroom coverall suit to ensure proper coverage of your skin and clothing.
Enhancing Safety
Cleanrooms are indispensable in enhancing the safety of pharmaceuticals, medical devices, and biotechnology applications by preventing contamination. Regulatory compliance in these environments ensures adherence to strict cleanliness standards, essential for protecting patient health and ensuring product integrity. Filtration systems, including high-efficiency particulate air (HEPA) filters, are crucial for maintaining air quality. Moreover, HVAC systems in cleanrooms are optimized to regulate temperature, humidity, and air quality, thereby meeting the critical requirements of both biotech and pharmaceutical industries.
Meeting Regulatory Compliance
Biotech cleanrooms must adhere to ISO 14644-1 classifications, often meeting Class 5 or stricter standards, to ensure cleanliness during the development of new treatments. Strict gowning protocols are in place to prevent contamination by personnel. Cleanroom coveralls, cleanroom lab coats and sterile cleanroom goggles are often part of the required apparel for cleanroom staff. Continuous environmental monitoring of temperature, humidity, and particle counts is vital for compliance with regulatory standards. The validation of cleanroom performance is critical in demonstrating adherence to both regulatory and industry standards, ensuring air cleanliness levels align with classification requirements set by authorities like the FDA. Wiping down all surfaces with lint-free cleanroom wipes is also required. Be sure to choose the correct wipe for your application. Polyester cleanroom wipes are often the main choice for ISO4-5 Class 10-100 environments. Nonwoven lint-free wipes are the go-to wipe for less strict more general cleanroom wiping. Cleanroom Connection offer a complete source of cleanroom wipes for all ISO levels, and their cleanroom experts can offer free samples as well as recommend the correct cleanroom wipe.
Applications of Cleanrooms in Biotechnology
Cleanrooms are vital in biotechnology for producing biologics such as vaccines, gene therapies, and monoclonal antibodies. These processes require a sterile environment to prevent contamination. Controlled environments in cleanrooms support crucial operations like cell culture and fermentation in biotech manufacturing. Various cleanroom types, such as Barrier Isolators and Hard-Wall Cleanroom Suites, meet specific production requirements while maintaining high sterility levels. Air quality is managed using HEPA filtration systems, which achieve 99.99% efficiency on 0.3 micron particles. This significantly reduces contamination risk, ensuring product quality and safety.
Medical Biotechnology
Medical biotechnology relies heavily on cleanroom environments to maintain product safety and efficacy, particularly for diagnostics, vaccinations, and medicinal therapies. These cleanrooms are essential for research in areas like cancer studies, gene therapy, and stem cell research, where contaminant control ensures patient safety and research accuracy. Medical biotechnology and life sciences cleanroom supplies are vital to regulation compliance. Cleanrooms must adhere to industry-specific regulations to safeguard sensitive processes and prevent defects, ensuring the integrity of medical products. By eliminating contaminants, cleanrooms in medical biotech safeguard patient health and maintain product integrity.
Agricultural Biotechnology
In agricultural biotechnology, cleanrooms are crucial for developing and testing products, ensuring they remain contamination-free. This sector enhances food security and agricultural efficiency by using clean environments to support research and production of food and non-food items like biodegradable materials and biofuels. Indoor farming and indoor hydroponics cleanroom supplies are often used in these types of cleanrooms to ensure cleanliness. Cleanrooms in this field maintain high research integrity and quality through strict environmental controls. They typically adhere to ISO 14644-1 classifications to assure cleanliness standards are met, contributing significantly to food safety and agricultural advancement.
Environmental Biotechnology
Environmental biotechnology uses cleanrooms to examine biological samples and ensure environmental safety. These environments help test products for safety and facilitate environmental monitoring and cleaning processes. Cleanrooms mitigate contamination risks in studies assessing human impact on environmental quality, maintaining strict cleanliness for accurate research outcomes. By supporting the development of solutions for environmental monitoring and improvement, biotech cleanrooms play a crucial role in addressing environmental issues effectively and ensuring a safe natural environment.
Modular Cleanroom Design
Modular cleanrooms are meticulously designed to offer a sterile environment crucial for various laboratory tasks, especially in life science operations. They are instrumental in minimizing contamination risks for processes such as PCR and are ideal for sterile preparations in hospital pharmacies. These cleanrooms support a wide range of industries, including medical devices, pharmaceuticals, biotechnology, and microbiology, by maintaining safe, contaminant-free conditions.
Customizable modular cleanrooms cater to industry-specific standards and requirements, often meeting stringent ISO classifications. Advanced laminar flow systems draw ambient air through HEPA filters to create a controlled environment that significantly minimizes contamination risks. This adaptability ensures modular cleanrooms can accommodate sensitive processes across various sectors.
Benefits of Flexibility
Flexibility in cleanroom design allows companies to reconfigure manufacturing and office layouts to meet evolving needs efficiently. Modular cleanrooms are particularly beneficial for both startups and established enterprises by facilitating rapid expansion while keeping costs in check. They offer flexible leasing terms, enabling biotechnology companies to optimize operational efficiencies and reduce financial commitments.
Adapting cleanroom facilities to meet various regulatory compliance standards is essential in the biotech field. The customizable nature of modular cleanrooms permits alignment with specific industry requirements via tailored design and regulatory consulting, ensuring compatibility with emerging technologies and manufacturing processes.
Efficient Design and Build Process
Biotech cleanrooms are required to meet stringent ISO Class 5 standards or better, demanding high air change rates of 40 to 80 feet per minute. Hardwall construction is standard, providing superior contamination protection and easier maintenance compared to softwall setups. Compliance with ISO 14698 is often necessary to effectively manage biocontamination risks.
These cleanrooms incorporate high-performance HVAC systems for precise temperature and humidity control, creating a stable and clean environment for sensitive operations. Multi-stage HEPA filtration systems and efficient laminar airflow patterns are crucial for maintaining high air quality and strict cleanliness standards, crucial for maintaining product quality in life science industries.
Critical Components of Cleanroom Facilities
Biotech cleanroom facilities are integral to maintaining a contamination-free environment crucial for sensitive processes in the life sciences industry. The most common type in biotech, the Hard-Wall Cleanroom, is built with materials that prevent corrosion and particle shedding, achieving all ISO classifications, including Class 1. Key systems like filtration and pressurization are vital for ensuring a clean, controlled, and compliant environment, using multi-stage HEPA filtration and laminar airflow to uphold high air quality standards. Various cleanroom types, such as Barrier Isolators and Mobile Cleanrooms, are designed to fit specific cleanliness needs, catering to the diverse processes within the pharmaceutical and biotech sectors.
HVAC Systems
In biotech cleanrooms, HVAC systems are essential for maintaining temperature, humidity, and air quality, which are crucial for a controlled environment. These systems are designed to deliver more air with specific airflow patterns and high-efficiency filters, surpassing standard systems. Effective HVAC design helps meet strict ISO classifications, supporting efficient operations and protecting sensitive processes by maintaining high air quality, minimizing contamination risks through mechanisms like laminar airflow and advanced filtration.
Protective Apparel
Protective apparel is crucial in maintaining clean and aseptic conditions within biotech cleanrooms. Cleanroom Connection provides tailored single-use disposable apparel designed to uphold controlled environments in life sciences applications. Quality-tested gloves are available for ISO Class 3, 4, or 5 cleanrooms, ensuring compliance with rigorous cleanliness standards. Proper use of protective gear prevents contamination from personnel, preserving product integrity in pharmaceutical and medical device production. Choose from cleanroom shoe covers, cleanroom face masks, cleanroom gloves and other protective lint-free apparel from Cleanroom Connection to ensure your cleanroom is particle and contamination-free.
Environmental Monitoring Systems
Environmental monitoring systems are vital for tracking parameters like temperature, humidity, particle counts, and pressure differentials, ensuring cleanroom integrity. Systems like CleanConnect offer 100% traceability and compliance, crucial for environmental monitoring in cleanrooms. Regular monitoring helps detect air and surface contamination, maintaining controlled environments in biotech. Compliance with standards like ISO 14644-1 is supported by particle counters and other devices, emphasizing the need for effective environmental monitoring.
Challenges in Maintaining Cleanrooms
Maintaining cleanrooms involves adhering to complex regulatory standards such as ISO 14644, ISO 13485, and FDA regulations. These standards demand strict control over cleanliness levels, adding operational complexity for operators. Premium HEPA filters must meet rigorous efficiency criteria to keep airborne particles at bay, proving crucial yet challenging.
Designing and equipping cleanrooms to meet contamination control requirements necessitates precise execution to prevent contamination risks. Cleanroom types vary, such as HardWall cleanrooms and Barrier Isolators, requiring careful analysis of each’s specific operational needs. Specialized systems, including HVAC, must be meticulously integrated to achieve controlled environments regarding cleanliness, temperature, and humidity.
Contamination Risks
Cleanrooms are pivotal in reducing contamination risks during various life sciences processes, including manufacturing and packaging. The goal is to eliminate contaminants like dust and microbes, ensuring the integrity of medical devices and pharmaceutical products. Adhering to strict regulations is essential to protect sensitive processes and prevent defects.
Efficient cleanroom design and operation are critical in minimizing contamination risks. Failure to maintain a pristine environment can lead to defects, making quality assurance an urgent priority. Addressing unclean environments is the simplest initial step in mitigating contamination threats.
Compliance with Standards
Biotechnology cleanrooms must comply with ISO 14698, emphasizing biocontamination control to maintain product safety and regulatory compliance. Life sciences companies adhere to USP Standard 797 and FD&C Act Sections 503A and 503B for compliant drug compounding and manufacturing. Cleanroom solutions aid life sciences industries in meeting these stringent standards for a controlled product environment.
Pharmaceutical cleanrooms are classified by the FDA based on air cleanliness levels using particle counts, adhering to ISO guidelines. Most biotech cleanrooms must meet or surpass ISO 14644-1 Class 5 standards to ensure ultimate cleanliness for developing critical treatments and compounds. Compliance is vital for maintaining product quality and safety in the life sciences sector.
Future Trends in Cleanroom Technology
Cleanroom technology is poised for significant advancements, with a strong focus on integrating advanced control and monitoring systems. These systems will enable real-time visualization and assessment of critical parameters, such as air humidity and temperature, ensuring optimal conditions for sensitive processes. Additionally, innovations in cleanroom design will aim to enhance mechanical performance, soundproofing, and ergonomic features, ultimately improving operational efficiency and worker comfort. To minimize contamination risks, the adoption of effective air shower systems will become more prevalent, maintaining cleanliness even in high-traffic environments. Sustainability will also play a crucial role in shaping future cleanroom technologies, driving efforts to optimize energy consumption, material usage, and waste generation. The biotechnology sector will likely see increased use of modular cleanrooms, offering flexible and scalable solutions that can be rapidly deployed to meet diverse production needs.
Innovations in Cleanroom Design
Biotechnology cleanroom design is evolving to include flawless layouts and specialized systems that prioritize contamination prevention. HardWall cleanrooms are becoming the preferred choice in biotech settings due to their ability to meet all ISO cleanroom classifications and support operations demanding high cleanliness standards. These cleanrooms are equipped with advanced control and monitoring systems, allowing real-time visualization and regulation of critical conditions like air humidity and temperature. Air shower systems play a key role in minimizing dust particle transfer from personnel, which enhances contamination management. Pressurization control and effective filtration of both indoor and outdoor air are vital to eliminating cross-contamination vulnerabilities.
Evolving Regulations
The life sciences industry operates under stringent regulatory frameworks to ensure product safety and environmental integrity. The FDA classifies pharmaceutical cleanrooms based on air cleanliness, measured by airborne particles per cubic meter. Compliance with international standards, such as ISO 14644 for air cleanliness and ISO 14698 for bio-contamination control, is essential for maintaining cleanroom operations. Regulatory changes, including those from the EU Medical Device Regulation (EU MDR), significantly impact life sciences companies by affecting their operational and compliance requirements. As the biotechnology landscape evolves, companies must frequently adapt to regulatory changes to maintain competitiveness and ensure safety in product development.